Hydrogen Embrittlement from Welding
scroll down for video
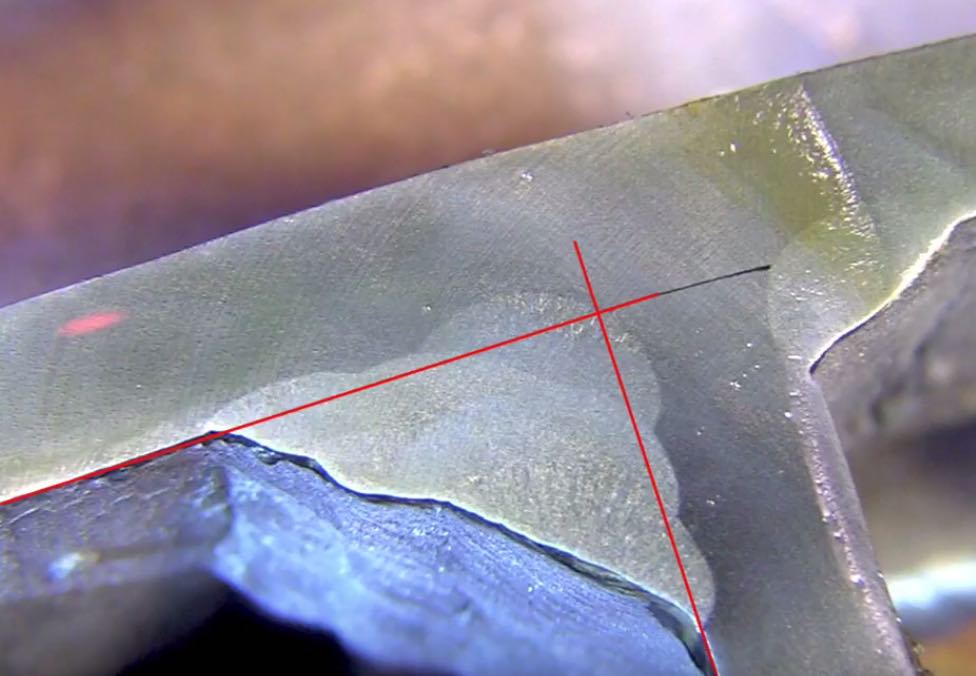
Hydrogen embrittlement from welding happens when hydrogen atoms enter the metal during or after welding, making the metal weaker and more likely to crack. Here’s a simple breakdown of what happens:
How Does Hydrogen Get Into the metal?
During welding, hydrogen can come from several sources:
- Moisture: Water (from humidity, condensation, or damp electrodes) can break down into hydrogen under the extreme heat of welding.
- Oil, Grease, paint, or Contaminants: Dirt, rust, or oil on the metal surface can release hydrogen when exposed to welding heat.
- Flux and Shielding Gas: In some welding processes, damaged flux or impure/insufficient shielding gas can allow hydrogen to enter.
What Does Hydrogen Do to the Metal?
When hydrogen atoms enter the hot, molten weld pool, they dissolve into the metal grain boundaries.
As the weld cools and solidifies:
- Hydrogen Gets Trapped: The metal cools quickly, and the hydrogen atoms don’t have enough time to escape. They become trapped inside the solid metal.
- Creates Weak Spots: These trapped hydrogen atoms form tiny pockets or stress points in the metal.
- Causes Cracking: Over time, the trapped hydrogen weakens the metal, especially in high-stress areas. This can cause delayed cracking (sometimes called "cold cracking"), which may not appear immediately but develops hours or days after welding.
What Are the main problems with hydrogen embrittlement from welding?
- Brittleness: The metal becomes less flexible and more prone to breaking.
- Cracks: Small cracks may form in the weld or heat-affected zone (HAZ), reducing the overall strength of the joint.
- Failure: In extreme cases, hydrogen embrittlement can lead to complete failure of the weld, especially in critical applications like pressure vessels, pipelines, or structural components.
What Metals Are Most Affected?
- High-strength steels are the most vulnerable to hydrogen embrittlement due to their hardness and lower ductility....some examples of high strength steels are A36, A572, A992, A500, A514, A516, A242, A588, A709, A913, 4130, 4140, 4340, and HY80.
- Aluminum and stainless steels are less likely to experience hydrogen embrittlement.
How to Prevent Hydrogen Embrittlement In Simple Terms
- Cleanliness: Ensure the metal surface is free of moisture, rust, oil, and other contaminants.
- Dry Electrodes: Use dry welding rods or electrodes, and store them properly to prevent moisture absorption. Remember that low hydrogen electrodes like 7018 must be stored in a rod oven at specified temperatures to ensure low hydrogen compliance.
- Preheating: For some metals, preheating can help reduce hydrogen absorption by slowing the cooling process and allowing hydrogen to escape.
- Post-Weld Heat Treatment (PWHT): After welding, heat the metal to a specific temperature to release any trapped hydrogen.
- Use Low-Hydrogen Welding Processes: For example, low-hydrogen electrodes (like 7018) or proper shielding gas can minimize hydrogen in the weld puddle.
Think of hydrogen embrittlement like tiny little strongmen in the metal caused by hydrogen sneaking in during welding. These tiny strongmen try to escape as the metal cools and push against the grains like the pictures you see of samson pushing against the pillars....making the metal weaker and more likely to fail, especially over time. The best way to prevent this is to keep everything clean, dry, and follow proper welding procedures.